Foraminiferans As Palaeoenvironmental Indicators.
About the author

Benauld
Introduction
Foraminiferans (hereafter referred to as Forams) are unicellular protists that inhabit the world’s oceans and range from 20µm to a few centimetres in size. They tolerate a wide range of salinities from esturine to fully oceanic (~ 35‰) but are NOT found in freshwater. They can be either planktonic or benthic, do not photosynthesise so inhabit various depths, and are active predators.
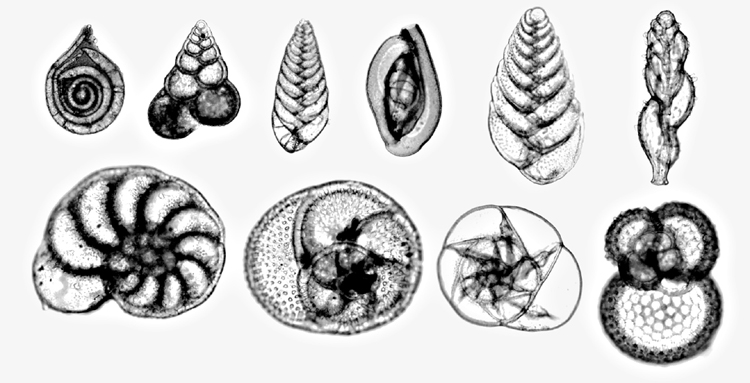
(Photo Randolph Femmer/NBII.)
Forams comprise an organic body encapsulated in a mineralised “shell” properly called a test. There are three types of Foram test:
- Calcium Carbonate.
- Silica.
- Agglutinated – shell fragments and other particles “glued” together by the Foram.
Controlling factors
Any assemblage of Foram populations is controlled by differing combinations of the following factors:
- Salinity.
Forams are found at their highest diversity in normal oceanic waters. They can be described as euryhaline – those species capable of living in a wide range of salinities, oligohaline – those which live in low salinities, or stenohaline – those which can survive only in an extremely narrow range of salinities.
In any salinity which is less than optimum, for example increased salinity in hypersaline lagoons, or reduced salinity in estuaries and deltas, Forams are often found in low diversities but high abundance. - Temperature.
Forams have a worldwide distribution from polar waters in the north and south, to tropical waters at the equator. However, individual species often tolerate only a very narrow temperature range.
Furthermore, warm and cold water assemblages are distinguishable by differences in the direction of test coiling. Warm water species usually exhibit a dextral (or right-handed) coil, cold water species tend to be characterised by the reverse a sinistral (or left-handed) coil. However, only planktonic species can provide useful information for past seawater temperatures, this is because variation in climate only causes the surface dwelling communities to change, bottom waters tend to remain at the same temperature, therefore benthic communities tend to remain equally stable. - Substrate.
This can be a major influence upon the preservation of individual Forams.
Fine-grained sediments: Silt and mud, (usually indicating low current strengths) are often comparatively rich in organic food particles; therefore there are often high numbers of Forams available for fossilisation. However, conversely the species here tend to be smooth and thin-walled and fossilise poorly.
Sandy: sand, (usually indicating higher current strengths) contains less food, and therefore low numbers of Forams. However, Forams here tend to be thick-walled and ornamented which increases the chances of preservation.
In hard substrate areas, for example bare rock, Forams are generally found attached by mucus threads. - Light.
Light only indirectly affects Forams by controlling the availability of their food source, for example zooplankton and algae. As a result the majority of Forams tend to live in the photic zone where food is commonly available. Nevertheless, there are species which live in complete darkness inhabiting the abyssal plains. - Depth.
Depth has no direct affect on Forams, but does have an influence on their food source (see above).
A typical sample will contain both benthic and planktonic species, however only benthic forms provide information about water depth, as planktonic forms simply fall through the water column upon death. - Oxygen.
Some species of Foram are able to withstand very low oxygen levels, even to the point of anoxia. Often populations here are once again characterised by high abundance but low diversity. - Dissolved CaCO3.
CaCO3 is more easily dissolvable in cold water, therefore cold water species tend to have sillica or agglutinated tests. - Warm interglacial conditions = Low 18O/16O Ratios.
- Cold glacial conditions = High 18O/16O Ratios.
- Due to the widespread distribution of many planktonic Forams they make excellent zone fossils for use in correlating long ocean cores.
- Provide depth indicator species and assemblages.
- Provide temperature indicator species and assemblages, in addition to test coiling direction.
- The presence or absence of CaCO3 tests provides information relating to changes in the calcite compensation depth.
- 18O/16O ratios in Foram tests provides detailed temperature information which can be correlated closely with ice core data.
Other Factors
Calcite compensation depth (CCD)
The CCD is the depth in the sea at which the rate of dissolution of solid calcium carbonate equals the rate of supply. Surface ocean waters are usually saturated with calcium carbonate, so calcareous materials are not dissolved. At mid-depths the lower temperature and higher CO2 content of seawater cause slow dissolution of calcareous material. Below about 4500 m waters are rich in dissolved CO2 and able to dissolve calcium carbonate readily. Carbonate-rich sediments are common in waters less than 3500 m depth, but are completely absent below about 6000 m. (Andrews et. al., 2004) and as a result in any ocean greater than circa 3500 metres in depth there is a comparative reduction in the number of Foram tests. Only silica and agglutinated tests survive to become preserved.
Oxygen Isotope Ratios In Foram Shells
Oxygen is incorporated in sea water in the form of H2O, the ratio of which is 1:500 for 18O:16O respectively in Standard Mean Ocean Water. As a result water can be described as being either “heavy” or “light” due to variations in this ratio. Heavy water has a tendency to remain in sea water whilst light water is preferentially evaporated. Consequently, warm conditions, with more heat energy available for evaporation, removes more heavy water molecules.
Therefore:
Forams incorporate these oxygen isotope ratios in their tests as CaCO3. These ratios are measurable and as a result Forams make good palaeo-thermometers.
Summary: Forams As Indicators of Climate Change.
References and further reading
J. E. Andrews et. al., 2004. An introduction to environmental chemistry .
 GeologyRocks
GeologyRocks